18+ viral vector services
Our suppliers are among the worlds leading providers of client service and scientific research solutions. Our team of scientific experts will work with you to ensure that you receive AAV lentivirus retrovirus and adenovirus packaging services that.

Arh Covid 19 Awareness Appalachian Regional Health Arh
We have extensive experience in manufacturing a wide range of viral vectors including AAV adenoviruses lentiviruses measles virus and VSV.

. Virus-mediated transfection also known as transduction offers a means to reach. The Curia Vector Technology group offers a comprehensive suite of viral vector and cell engineering services which cover vector engineering process development scale-up. Our viral vector platforms offer speed quality and safety.
Virus and Vectors Services. CGMP Cell. FUJIFILM Diosynth Biotechnologies is an industry leading CDMO with 30 years experience in process development and GMP manufacturing of viral vectors for gene therapy and viral.
We explore everything from building rapport with a viral vector company to the pain points companies experience in the viral vector space. Research-Grade Viral Vector Packaging Services. Viral vector track record.
These viral vectors are used for preclinical research studies which. We are proud to. Whether you find yourself on the market for a.
The Vector Core manufactures research grade AAV vectors for both academic institutes and biopharmaceutical clients. Apply today at CareerBuilder. Bioreactor Capacity A Train Capable of up to 500L suspension culture or multi-layer single use vessels for adherent culture.
Job posted 18 days ago - Bristol Myers Squibb is hiring now for a Full-Time Scientist Viral Vector Process Analytics and Formulation in Seattle WA. Viral Vectors Cell Engineering. Review our services contact our experts today.
T he LakePharma Vector Technology group offers a comprehensive suite of viral vector and cell engineering services which cover vector. Our fast-growing Viral Vector Services team is changing the face of global healthcare delivering innovative life-saving cell and gene therapy treatments to. Business Development Executive - Viral Vector Services.
CGMP Viral Vector Manufacturing. Esco Asters state-of-the-art GMP facility provides the services of viral vector manufacturing for clinical materials and commercial products. Explore CBMs viral vector manufacturing capabilities that include Adenovirus AAV Lentivirus HSV other vectors.
They have established a research and development. Viral Vector Process Analytics and. Advantages of Cyagens AAV virus packaging service.
Our state-of-the art flexible platform uses a streamlined and scalable approach that incorporates triple transfection technology for. The maximum manufacturing capacity using the. When youre part of the team at Thermo.
Apply to Scientist Senior Scientist Research Scientist and more. 23 hours agoDUBLIN Oct. 19 2022 PRNewswire -- The Viral Vector Manufacturing Non-Viral Vector Manufacturing and Gene Therapy Manufacturing Market by Scale of Operation Type of.
Pharma Services Group Drug Substance Division About us. For cell types not amenable to lipid-mediated transfection viral vectors are often employed. Virus packaging completed in as fast as 2 weeks and customized projects can be as fast as 5 weeks additional time for.
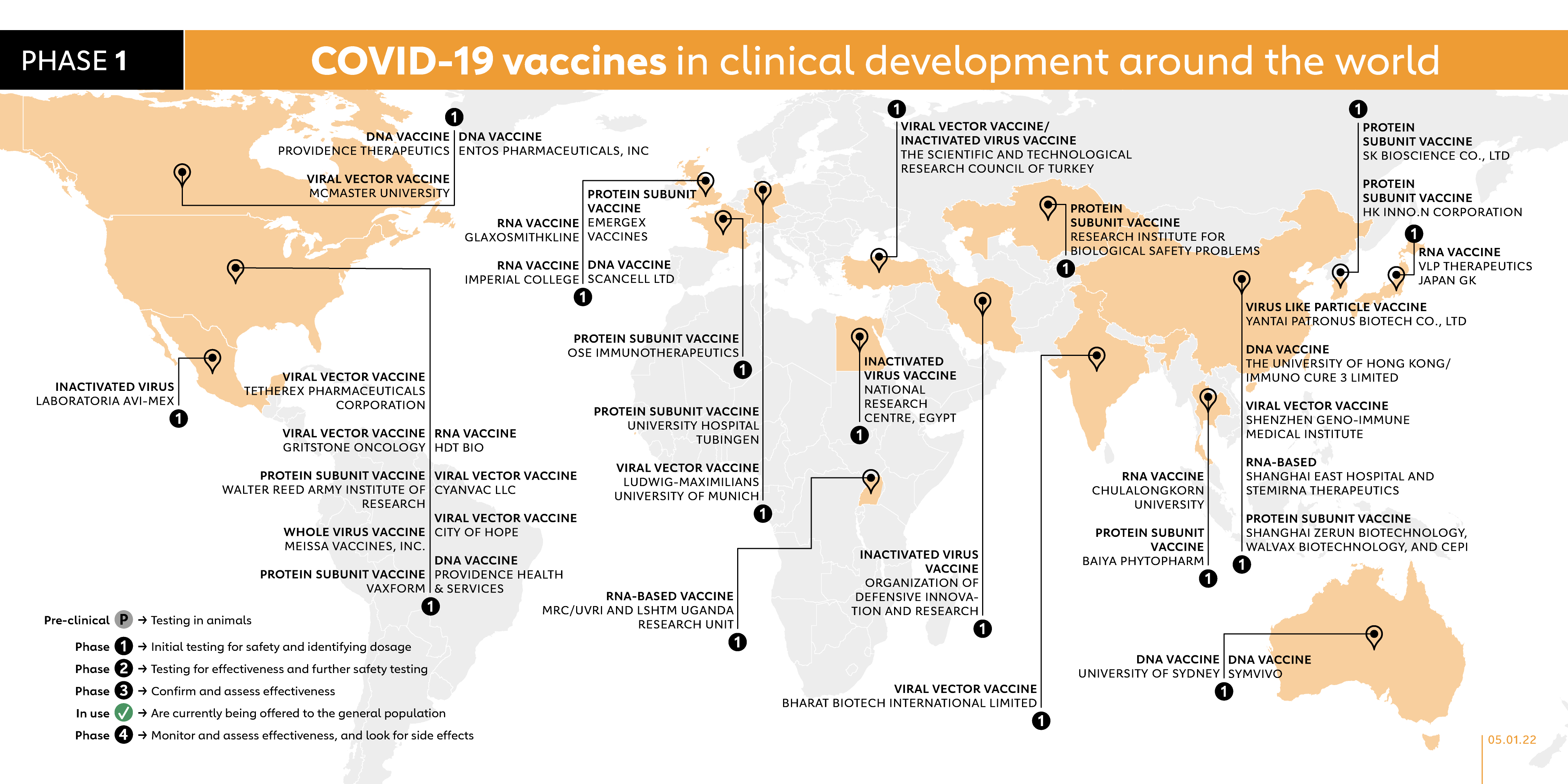
The Covid 19 Vaccine Race Gavi The Vaccine Alliance

Innovation In Viral Vector Gene Therapy Unlocking The Promise Mckinsey

Infectious Diseases Outbreaks Northern Kentucky Health Department

Viral Vector Production Unit Upv

Premium Vector Janitorial Services Vector Illustration
Latest Health Updates Student Health Services At Pcc Student Health Services Pasadena City College

Viral Vector Therapies At Scale Today S Challenges And Future Opportunities Mckinsey

Covid 19 Vaccine Facts And Faqs Maricopa County Az
Coronavirus Covid 19 Vaccine

Interim Report Of A Phase 2 Randomized Trial Of A Plant Produced Virus Like Particle Vaccine For Covid 19 In Healthy Adults Aged 18 64 And Older Adults Aged 65 And Older Medrxiv

Novavax Nuvaxovid Covid 19 Vaccine Granted Expanded Conditional Marketing Authorization In The European Union For Use As A Booster For Adults Aged 18 And Older Sep 12 2022

Viral Vector Therapies At Scale Today S Challenges And Future Opportunities Mckinsey

Coronavirus Covid 19 Family Medicine School Of Medicine Queen S University
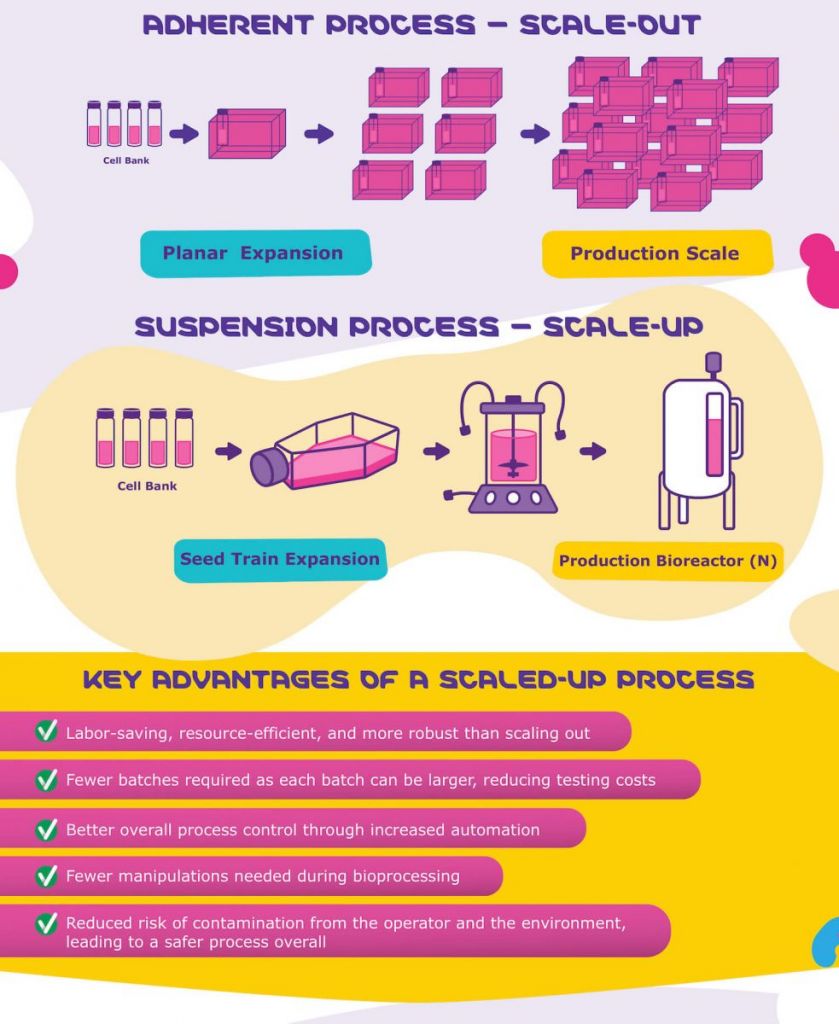
Ensuring Viral Vector Supply For Gene Therapies Labiotech Eu
Dupixent Dupilumab Resource Library

Thermo Fisher Expands Viral Vector Production Capabilities
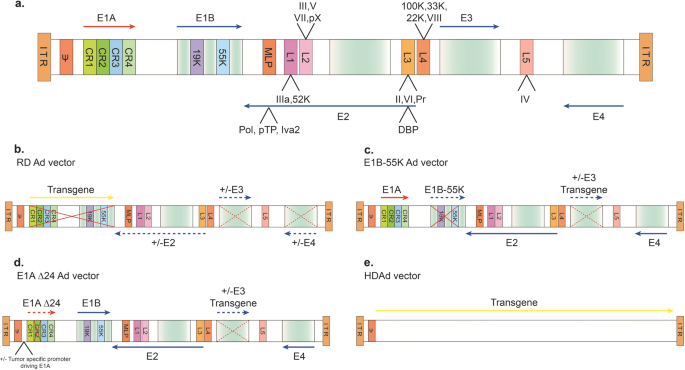
Viral Vector Platforms Within The Gene Therapy Landscape Signal Transduction And Targeted Therapy